You are here
- Home
- Astrobiology – a science, a passion, and inspiration for science fiction
Astrobiology – a science, a passion, and inspiration for science fiction

I am honoured to be able to kick things off on this blog. From now on, we will have a post each week from one of our team members, and if you keep following us, you’ll learn a lot about the different things we do.
You will hear from scientists, from technicians, and from the heroes who do our administration, too. You will see blogs written by all the team, from lawyers to physicists, from PhD students to professors… in no particular order. We hope you enjoy the insights we can give you through this, and if you want to contact us, follow us on twitter @Astrobiology_OU
As a matter of introduction, I am one of the associate directors of AstrobiologyOU. If you wonder, how I came to do Astrobiology then I have to first confess that until about 10 years ago, I didn’t even know that’s something I could apply my research to. Why? Well, I am not a biologist, I am a mineralogist – which is kind of a specific breed of Earth scientists. I care about what happens, when minerals react with water, and what remains as evidence when they are done with that and the water is long gone.
Confused? Well, then let’s think of this in another way: There are rocks that form from magma – you can think of the volcanoes in Iceland or Hawaii, for example. If that happens, a certain set of minerals form: feldspars, pyroxenes and olivine. This rock is called a basalt. If that basalt has crystallized and cooled, it will be exposed to all the processes that happen later in history. Let’s assume the volcano erupts again, at which moment new heat is put into the subsurface at that location. Let’s also assume there is some water (not hard to imagine in Iceland or Hawaii, right?), then that water will get heated by the magma from the new eruption. It will be pressed through cracks of existing rocks, and it will react there.
But rarely do we get to watch this in person (and I am convinced it’s not a good idea to be around 1000 °C hot, molten rock that meets water and instantly turns some of that into steam, anyway). As a mineralogist, it is much more common to visit a rock formation millions of years after the processes have happened. Then we are faced with a crime scene: We know how this rock – in our example the basalt – should look. But we see the result of the water-rock reactions. We can observe the cracks, we might see places that look like the original rock has been dissolved, and we see newly formed crystals. Like a detective, we get our hand lens out, and we take samples. We then go back to the laboratory and investigate those with a microscope and more complicated analytical techniques, too. So, we can piece together all the evidence, but that still doesn’t answer the question ‘Who’s done it?’.
What we want to know – the answer to the ‘who’s done it’ question is what temperature the water had, what its acidity was, and if it carried any other dissolved salts when it entered the rock. These are not trivial questions to answer, because the water might have brought elements with it, for example sodium, if it was seawater (again, not hard to imagine in Iceland or Hawaii, right?). But the water will also inevitably have carried away some dissolved salts, too, and probably deposited them somewhere else, in a cooler environment. To find out what really happened, I use a technique called ‘thermochemical modeling’. What is behind this is a computer code that tries to find out in what combination of minerals the free energy of a system is minimized. It does that by solving many equilibrium equations at the same time. I personally use ‘CHIM-XPT’, which is written by Prof. Mark Reed (University of Oregon) and his group, but – as they say – ‘other brands exist’. I have to understand as much as possible of the rock that was there before the water came – and as much as I can about “the crime scene”. With that, I can find out, the temperature of the water, it’s pressure and also the amount of water that is needed to ‘match’ the crime scene. Was there a lot of water? Or very little? That’s expressed by the water to rock ratio – W/R. I include a diagram here – my colleagues all just call it ‘the wiggly line plot’. See, if you agree:
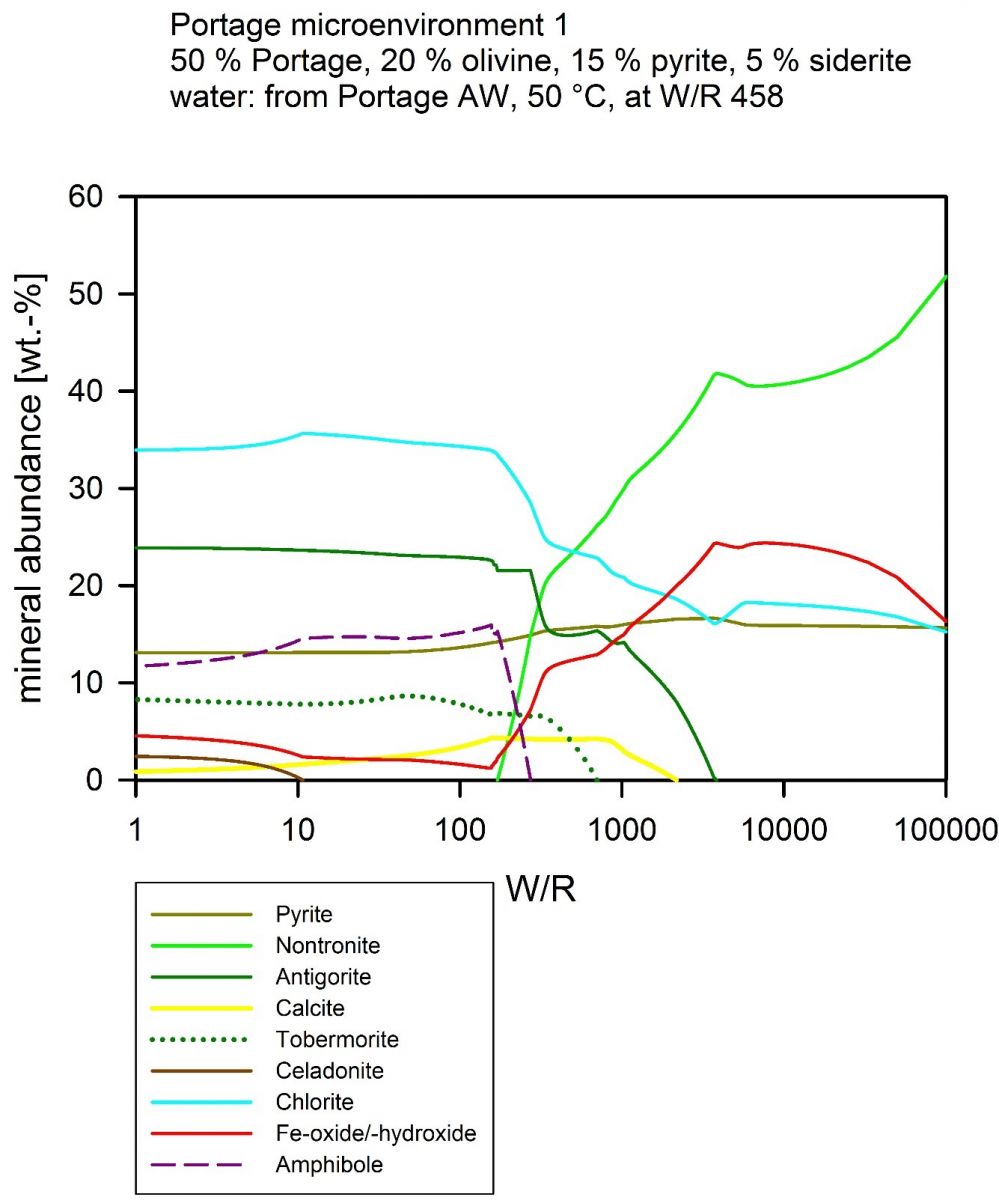
This is one of my plots that I made from a Martian rock composition, which the Curiosity Rover has found at Gale Crater. The model was done at 50 °C. If you focus on the light and dark green lines first, which are the ‘Nontronite’ and ‘Antigorite’ minerals according to the key below the figure, and also add the turquoise line (‘Chlorite’ according to the key) to the three things you are looking at, then you are looking at the minerals that are together making up the ‘phyllosilicates’ – or sheet silicates. Some are typical clay minerals – the nontronite is an example – and could form at room temperature, others need a little higher temperatures, the antigorite is an example. What you see is that if there is very little water in the system, antigorite forms, that’s the left part of the diagram, where W/R is low. If there is more water, nontronite forms. And in that area, there also is that prominent red line… it’s iron oxide, in this case hematite – the mineral that makes Mars red.
To get to that diagram was about 3 weeks of work, staring at my screen for several hours each day, because I had to try different temperatures and settings, all of which tell me details of the answer to ‘Who’s done it’. In the end, I need to see if these results match what has been found on Mars. If you have followed the MSL Curiosity Rover mission, you’ll know that clay minerals such as nontronite have been found a lot – and hematite has as well, which makes the right side of the diagram a potential answer to the question!
And because I love that mission, and even get to work on it as a member of the science team, I leave you with this beautiful image of a very red drill hole on Mars (above), the Duluth drill hole. I also want to point you to the MSL blog if you want to know more about the mission (full disclosure, I occasionally write those mission blogs, too). And if you want to find me on twitter, it’s @SPSMars. For those, who want to dig deeper into the science, I have – with many colleagues – written two peer reviewed publications of which the above diagram was just one on the way to the final answer. You can find both on the OU’s OpenResearchOnline, here, and here.

Author:
Dr Susanne P. Schwenzeris a Senior Lecturer at the Open University and is Associate Director of AstrobiologyOU